Program Synopsis
Designed to support vaping cessation among adolescents and young adults interested in quitting, this multimodal digital intervention (formerly known as This is Quitting) includes tailored interactive, automated text messages, an interactive website, and tailored email messages. Two studies conducted among adolescents and young adults showed higher 30-day abstinence rates.
Program Highlights
Program Materials
Preview and order the materials from the developer
Program Scores
The Need
As of 2024, about 10% of high school students and 5% of middle school students use tobacco, most commonly in the form of e-cigarettes (i.e., vaping). Nicotine is highly addictive, and young people are particularly susceptible to addiction. E-cigarettes expose young people to numerous toxic substances and health risks, such as exacerbations of asthma, bronchitis, and respiratory tract irritation. Among young people who vape, more than 35% also use other tobacco products. No tobacco product is safe: These products can contain cancer-causing chemicals, heavy metals, and dangerous ultrafine particles in addition to nicotine. They can be especially harmful to young people, as the chemical products can negatively impact brain development until around age 25.
Providing tailored digital cessation resources for individuals who use e-cigarettes—especially adolescents and young adults—can be a cost-effective strategy to reduce tobacco use and prevent long-term nicotine dependence.
The Program
EX Program is a tailored and interactive intervention delivered through automated text and email messages, a web-based quit plan, and an online community to support vaping cessation among adolescents and young adults aged 13 to 24 interested in quitting. The program is grounded in social cognitive theory, with elements that aim to establish perceived social norms and social support for quitting, support observational learning, and enhance behavioral skills and strategies.
Participants register for EX Program either by text message or through the program website, and they can then choose to engage by any combination of these methods (text, email, or website).
Those who opt to receive text messages can set a quit date and receive roughly 12 weeks of tailored messages based on their age, quit date, and type of vaping product they use. For up to 6 weeks prior to the quit date, messages address the harms of vaping and the benefits of quitting and aim to build motivation, reinforce self-efficacy, and provide cognitive and behavioral strategies for managing cravings and stress. For 8 weeks after the quit date, messages offer coping tools and mental health support (e.g., mindfulness training, self-care), breathing training, and information about a crisis text line. Messages also address nicotine replacement therapy, describing its utility and either encouraging the use of Food and Drug Administration-approved medication (for ages 18 and older) or consultation with a health care professional (for ages 13 to 17). Participants can text keywords (e.g., TIPS, STRESS) for immediate guidance around cravings/withdrawal, advice, inspiration, and stress management. They are also directed to relevant content on the program website and to the online peer support community. Many messages are written by other young people who have participated in the program, blended with expert-written evidence-based behavior change strategies.
The frequency of text messages varies across the 12-week period based on the quit date, with the highest frequency being three messages per day. Participants also receive monthly check-in texts and are asked to report their vaping use. Users without a set quit date receive 4 weeks of preparatory messages focused on increasing readiness and highlighting the risks and benefits of cessation. These individuals need to set a quit date to continue receiving messages after this 4-week period.
Email messages include tips and advice related to quitting and are personalized and tailored by participant’s quit status and vaping product type. They also encourage participants to engage with the content on the interactive website. Users who sign up for email support receive automated messages 2 weeks before and 2 weeks after their quit date, with periodic messages thereafter. Users without a set quit date do not receive quit-date-related messages, only general cessation content.
The studies reviewed for this summary evaluated the effectiveness of text messaging alone without the additional program components.
Community Preventive Services Task Force Finding
 This program uses intervention approaches recommended by the Community Preventive Services Task Force: internet-based cessation interventions (Tobacco Control) and mobile phone-based cessation interventions (Tobacco Control).
This program uses intervention approaches recommended by the Community Preventive Services Task Force: internet-based cessation interventions (Tobacco Control) and mobile phone-based cessation interventions (Tobacco Control).
Time Required
The standard materials to promote the program take about 10 minutes to download and print, though additional time (a couple of hours to weeks depending on capacity, mailing list, and network sizes) will be required to disseminate them widely.
For organizations interested in a customized version of EX Program with evaluation support, the implementation process typically takes about 6 weeks from the time consultation begins.
Intended Audience
The intervention is intended for young people aged 13 to 24 who vape and are interested in quitting.
Suitable Settings
The program is suitable for implementation online.
Required Resources
Required resources to implement the program include the following:
-- Truth Initiative website
The program is freely available to young people aged 13 and older and organizations across the United States. A customized version can be implemented for organizations. For costs associated with this program, click on Contact the Program Developer on the Program Materials page.
About the Study
Two randomized controlled trials were conducted to evaluate the effectiveness of a text message vaping cessation intervention (This is Quitting, now part of EX Program) among young adults aged 18 to 24 years (Study 1) and adolescents aged 13 to 17 years (Study 2). In both studies, eligible participants lived in the United States, had used e-cigarettes in the past 30 days, expressed interest in quitting within the next 30 days, and owned a mobile phone with a text messaging plan. Participants were recruited nationally through social media advertisements linked to the study website.
For both studies, the primary outcome was 30-day abstinence from vaping, measured 7 months after randomization via self-report. Participants were asked, “In the past month, did you vape at all, even a puff of someone else’s?” Participants were instructed to consider use of all nicotine-containing vaping devices (including JUUL, mods, and other e-cigarettes) when answering this question. The assessment was conducted via text message and email, followed by a phone call by study staff if needed.
Secondary outcomes included assessments involving the use of cannabis and combustible tobacco products (CTPs) 7 months after randomization. One outcome was 30-day abstinence from both e-cigarettes and cannabis, and another was 30-day abstinence from both e-cigarettes and CTPs.
Study 1: Young adults. Participants were randomly assigned to the intervention group (n=1,304) or to an assessment-only control group, which received no intervention (n=1,284). To optimize study retention, both groups received assessments by text message 14 days after randomization and then monthly thereafter for 6 months.
The average age was 20.4 years, and 48.4% were male. Participants reported that they were White (83.4%), Asian (4.8%), Black (1.5%), American Indian/Alaska Native (0.7%), multiracial (6.3%), and “other” (1.9%), with 1.1% not reporting. Further, 10.6% were of Hispanic ethnicity.
Study 2: Adolescents. Participants were randomly assigned to the intervention group (n = 759) or to an assessment-only control group, which received no intervention (n = 744). To optimize study retention, both groups received assessments by text message 14 days after randomization and then monthly thereafter for 6 months.
The average age was 16.4 years, with the majority of participants in 11th or 12th grade (72.8%), and 41.8% were male. Participants reported that they were White (62.7%), Black (10.2%), Asian (2.1%), American Indian/Alaska Native (1.5%), Native Hawaiian or Other Pacific Islander (0.4%), multiracial (18.5%), and “other” (8.7%). Further, 16.2% were of Hispanic ethnicity.
Key Findings
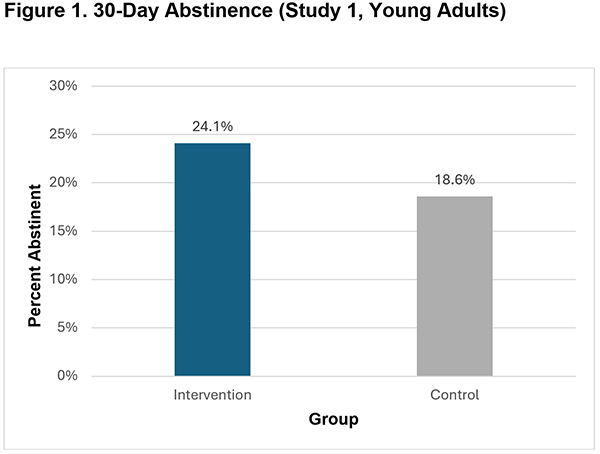
- A higher percentage of intervention group participants reported 30-day abstinence than control group participants at the 7-month follow-up (24.1% vs. 18.6%, p<.001).
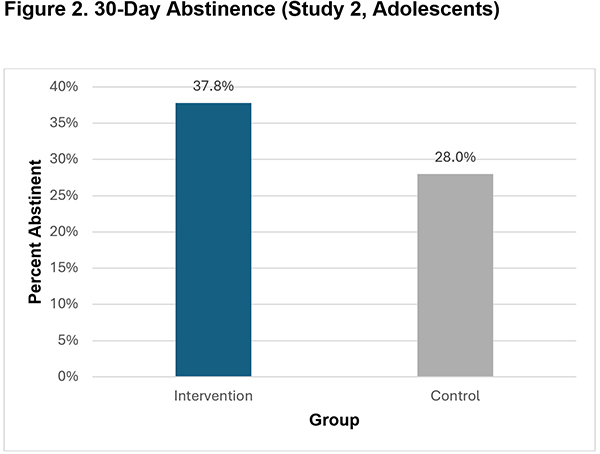
- A higher percentage of intervention group participants reported 30-day abstinence than control group participants at the 7-month follow-up (37.8% vs. 28.0%, p<.001).
Additional Findings
- A higher percentage of intervention group participants reported 30-day dual abstinence from both e-cigarettes and cannabis than control group participants at the 7-month follow-up (young adults: 17.9% vs. 13.3%; p = .007, adolescents: 38.5% vs. 25.0%; p < .0001).
- Among adolescents using both e-cigarettes and CTPs, a higher percentage of intervention group participants reported dual abstinence from both e-cigarettes and CTPs than control group participants at the 7-month follow-up (50.8% vs. 30.0%; p<.001).


